Alzheimer's Clinical
Trials Consortium
Our mission is to provide an optimal infrastructure, utilizing centralized resources and shared expertise, to accelerate the development of effective interventions for Alzheimer’s disease and related dementias (ADRD).
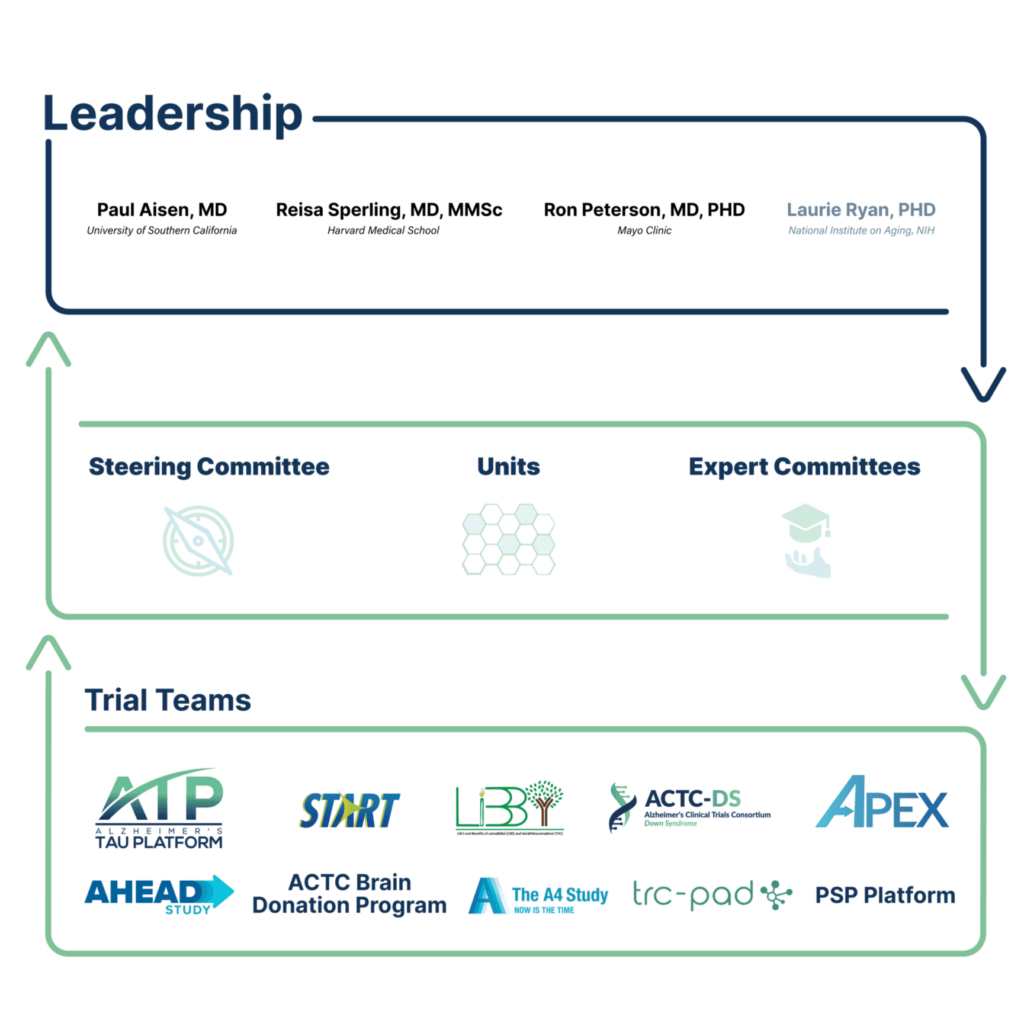
ACTC Organization
ACTC infrastructure includes expertise in study design and conduct, and full clinical trial management capabilities through our coordinating center and network of trial sites.

Efficient
Through centralization of resources and processes, we develop and utilize efficient solutions.
Innovative
We consider novel clinical trial designs, leveraging innovative cognitive, biomarker, neuroimaging outcomes, and data sharing.
Diverse
Our Education, Engagement, Access, and Accountability (ACT-E2A2) Unit promotes inclusion in all ACTC activities.
Experienced
Our investigators and sites have extensive experience conducting and enrolling ADRD clinical trials.
Collaborative
With each clinical trial’s lead investigator, we build a strong collaborative team to design and conduct trials.
ACTC Members
Member Sites
Our member sites are high performing and experienced clinical trial sites that receive infrastructure support from ACTC.
Units
Our dedicated Units are the driving force behind the day-to-day conduct of ACTC and oversee the development and execution of our clinical trials.



Committees
ACTC Committees are composed of experts in the field and advise investigators and study teams during the development and conduct of our clinical trials.




